
A decade of making medicines accessible
18 billion doses of treatment in 10 years
Annual report 2020

Annual report 2020


MPP licences have generated USD 1.96 billion in global health savings through the procurement of more affordable quality-assured medicines from MPP generic partners through an average price reduction
of 81%
relative to the originator price
Generic products facilitated by MPP have been distributed in 148 countries providing 49.71 million patient-years of treatment from January 2012 to December 2020
MPP’s impact is calculated and verified by KPMG




Since 2010, and the foundation of MPP, much has happened – dozens of negotiations on public health licences, hundreds of partnerships across sectors, billions of doses of treatment supplied through MPP’s licences, and much more. And behind all these successes are hard-earned lessons that we have gathered, one lesson at a time. Each of these 10 precious lessons, as reflected in our partners’ voices, has made our foundation stronger than ever.
Our vision is a world in which people in need in low- and middle-income countries (LMICs) have rapid access to effective and affordable medical treatments and health technologies.
Our mission is to increase access to, and facilitate the development of, life-saving medicines for LMICs through an innovative approach to voluntary licensing and patent pooling. We work with a range of partners — civil society, international organisations, industry, patient groups and governments — to prioritise and license novel and existing medicines and health technologies for people in these countries.




27.4 million people were accessing antiretroviral therapy in 2020, an increase of 2m since 2019
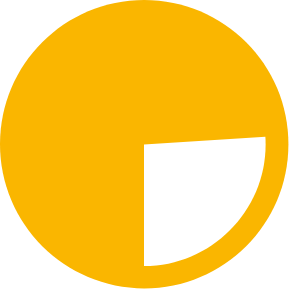 26% of adults
26% of adults
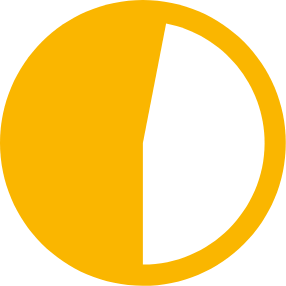 47% of children
47% of children
living with HIV still miss out on HIV treatment, of whom the vast majority live in low- and middle-income countries1
1UNAIDS, 2020 fact sheet (last accessed on 14 June 2021)



Access to hepatitis C treatment is improving but remains too limited.
In 2019,21%
of those living with the HCV infection knew their diagnosis.
Of those diagnosed with chronic HCV infection, 9.4 million people (62%) had been treated with DAAs by the end of 2019.
Much more needs to be done to achieve
80%HCV treatment target by 20302.
2World Health Organization, Global report on HIV, viral hepatitis and sexually transmitted infections, 2021





Multidrug-resistant TB (MDR-TB) remains a public health crisis and a health security threat. A global total of 206,030 people with multidrug- or rifampicin-resistant TB (MDR/RR-TB) were officially diagnosed and notified in 2019, a 10% increase from 2018. Ending the TB epidemic by 2030 is among the health targets of the Sustainable Development Goals (SDGs). To meet this target, faster-acting and better therapies to treat TB are urgently needed, particularly for MDR-TB4.
4World Health Organization, Fact Sheet, Tuberculosis, October 2020 (website accessed on 14 June 2021)

COVID-19, the disease that dominated the world’s attention throughout 2020, was declared a pandemic by WHO on 11 March 2020. Shortly after, MPP swiftly realised that equitable access to medicines and technologies for COVID-19, as they become available, will be a key factor in determining how effectively we deal with this pandemic. In consequence, MPP’s Board expanded the organisation’s mandate to COVID-19 on 31 March 2020.
The following days and months saw MPP charting the possible roles it could play in defeating the new coronavirus. By applying its tested voluntary licensing and patent pooling model, MPP could:
Help fulfil the need for huge volumes of treatments through its generic manufacturing partners
Leverage its broad partnerships towards increasing the geographical reach of effective technologies, especially in low- and middle-income countries
Aid in bringing down the prices of medicines by introducing multiple generic players and driving healthy competition among them
Ensure quality of generic versions of licensed health products
Complement direct efforts of originators and public health organisations towards leaving no one behind
Provide a sustainable model that does not rely on a philanthropic approach to access – one-off charities, philanthropic donations etc.



Initiating exploratory talks with patent holders of essential medicines for non-communicable diseases (NCDs), including cardiometabolic diseases and cancer, to gather industry perspectives and positions on the MPP model and explore potential willingness to partner with MPP to facilitate access to innovative products.
to improve access to affordable and high-quality diabetes medicines in LMICs.
to work closely in furthering the shared goal of promoting wide availability of quality, safe, effective and affordable essential medicines for better cardiovascular health.
this meant, in particular, the addition of biotherapeutics for NCDs that were added to the WHO EML in 20195.
With the World Heart Federation to improve access to NOACs (non-vitamin K antagonist oral anticoagulants) to make these life-saving innovations affordable and available in low-resource settings. The recommendations were published in the peer-reviewed journal Global Heart.
With the inclusion of several biotherapeutics in the WHO EML over the past three revisions, the WHO Expert Committee requested MPP to consider the application of its model to biotherapeutics. In that context, MPP started an assessment that will be concluded in 2021.

Long-acting regimens for the treatment or prevention of chronic illnesses, such as weekly oral pills or monthly patches, injectables and implants, are emerging as game-changers in healthcare. These pioneering innovations offer people a simpler yet effective way of administering medicines that frees them from daily pills, helps them stay on treatment and reduces the burden on health systems.
2020 started as an exploratory phase in the long-acting space for MPP, and by year-end, it became an integral part of MPP’s ongoing work.

MPP partnered with all three of Unitaid-funded long-acting projects – MedinCell, the University of Liverpool and the University of Washington
During the third Long-Acting Injectables and Implantables Conference in La Jolla, California (6-7 February 2020): MPP co-organised the side event with MedinCell to raise awareness about access to health technologies in LMICs.
MPP co-organised with Unitaid and WHO a satellite session titled “Harnessing access to long-acting technologies in low- and middle-income countries: are we on track to resolving the conundrum?”
Bringing its expertise to shaping the long-acting agenda in these areas, for which availability of extended-release drugs and formulations could profoundly affect treatment.
MPP ENGAGED with community representatives, treatment advocates, civil society members, the research and development community and the industry throughout the year to seek their perspectives on the needs and wants related to long-acting technologies and formulations, as well as potential bottlenecks that MPP could help address.


MedsPaL is a free resource that provides information on the intellectual property status of selected patented essential medicines in LMICs.
MedsPaL was launched in October 2016, focusing on medicines for three diseases: HIV, hepatitis C and tuberculosis. In December 2017, it was expanded to cover all patented medicines on the WHO EML. After the new WHO EML was released in July 2019, MedsPaL was updated to include patent information on the 18 newly listed medicines.



2020 brought MPP into the limelight on numerous occasions and in diverse contexts. With COVID-19 on the top of the global health agenda and MPP’s experience and model in access to medicines for other diseases, it was no surprise that a lot of MPP’s media mentions were related to COVID-19. MPP continued to bring even more life-saving treatments and access to HIV and hepatitis C medicines in 2020, and these achievements were newsworthy too! Here is a sample of news coverage on MPP in 2020.















You can also download the PDF here